Documenting for your QMS
Well managed documentation is the foundation of an effective quality management system (QMS).
The ISO 9001:2015 standard for quality is the world’s most broadly adopted management system. It outlines a defined set of quality principles, including customer focus, process approach, continuous improvement and evidence-based decision making. Interestingly, some of the most effective and value adding quality management systems are those with the simplest and most streamlined documentation.
Core elements of a QMS
There are nine core elements of a QMS:
- Quality objectives: defining quality objective includes pinpointing strategic goals and a purpose for the QMS. Objectives translate an organisation’s vision into practice by creating a link between customer requirements and specific, measurable, and attainable goals.
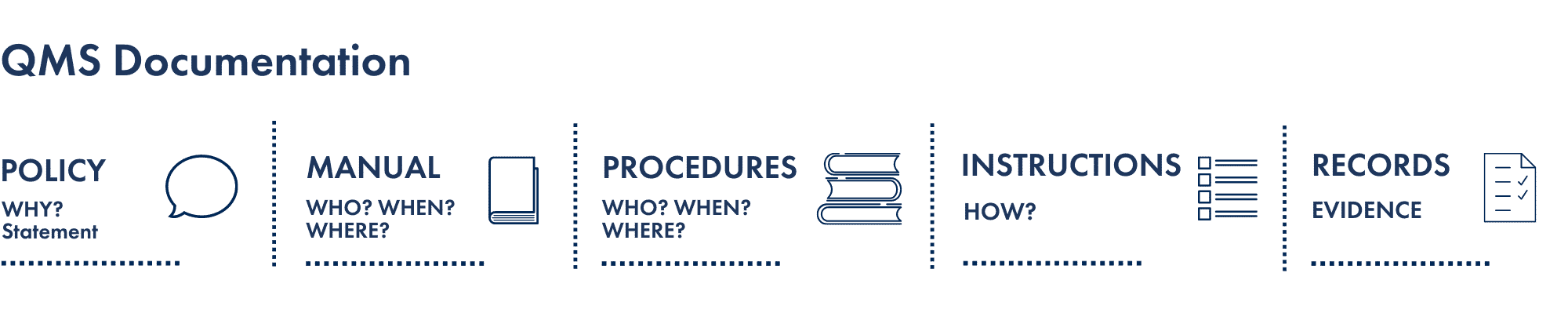
- Quality manual: the quality manual is the foremost documentation of a QMS. Typically, a quality manual will include the quality policy and objectives, details of the quality control system, staff roles and relationships, procedures, systems and any other related information that will assist the organisation in achieving its objectives. The Quality Manual should be easily understood, accessible and relevant to all staff. Therefore, It should not be too large or long winded.
- Organisational structure and responsibilities: a clear and updated model of the organisational structure and responsibilities of all individuals within the organisation. Documentation of structure and responsibilities should include visual guides such as flowcharts and clear documentation.
- Data management: data quality and availability are critical to the success of a QMS framework being able to drive continual improvement and preventative quality control activities. Organisations with ineffective data management practices can experience inconsistent product quality, operating inefficiencies, compliance risks, poor customer satisfaction, and low profitability
- Processes: quality management systems are inherently process-driven approaches to quality control and assurance. Standards for quality management require organisations to identify and define all processes within an organisation which use any resource to transform inputs into outputs. Virtually every responsibility in an organisation can be tied to a process
- Customer satisfaction with product quality: a QMS core component is the requirement for organisations to monitor customer satisfaction to determine whether quality objectives are achieved and if what the company is delivering is meeting the actual and perceived expectations of the customer.
- Continual improvement: continual improvement and adaptation are necessary for organisations to drive benefits with the QMS and maintain customer satisfaction. QMS dictate that continual improvement is an organisation-wide responsibility. In other words, all staff need to be working toward the improvement, fine tuning and betterment of the organisation.
- Quality instruments: each organisation will decide which methods are best suited to measure quality and the effectiveness of the QMS. Determining the most effective means of measurement is integral to the success of a QMS.
- Document control: the document or manual management system must contain all evidence necessary to objectively prove QMS performance, customer feedback and all internal activities such as audits and inspections. Document control is vital to the traceability and measurement of the QMS.
Why is QMS Documentation required?
ISO 9001:2015 is the world’s best known and most implement quality management system (QMS). Organisations implement ISO standards to demonstrate the ability to consistently provide products and services that meet customer and regulatory requirements. ISO 9001:2015 belongs to the International Standards Organisation family. It is one of thousands of standards aimed to standardise and identify quality organisations across the globe. ISO 9001:2015 is one of the most sought after and identified standards in the ISO 9000 series. Not to mention, it is the only standard in the series to which organisations can certify.
ISO9001 has the aim of enhancing customer satisfaction through the effective application of the system. This includes processes for improvement, and the assurance of conformity to both customer requirements and all applicable statutory/regulatory requirements.
All the requirements of the international standard are generic and are intended to be applicable to any organisation. This is regardless of its type or size, or the products and services it provides.
In a QMS, documentation is a core requirement for the overall success of the management system. The size and scope of the organisation will determine the level of documentation required, but it can consist of different types of documents, such as:
- Quality policy
- Quality objectives
- Procedures and instructions
- Risk register
- Strategic plan
- Records
The purpose and the benefits of the QMS documentation are multiple, including:
- Providing a clear framework of the operations in an organisation
- Allowing consistency of processes and better understanding of the QMS
- Providing evidence for achievement of objectives and goals
When designing QMS documentation, you should focus on efficiency and create processes and documents that are applicable in your organisation.
Implementing Effective Documentation
Good documentation will greatly improve your organisations effectiveness and efficiency by ensuring that your QMS has a strong foundation.
Effective documentation is easily achieved by following the 5 basic steps:
- Identify Documentation: identify all your processes and the interaction between them by mapping the various core processes and the relationship each one has with all stakeholders inside, and outside, the organisation. Analysis of the processes should then be used to determine the amount of documentation needed for the QMS. QMS standards will determine mandatory documents and procedures to cover each section of the standard that applies to your business. The complexity of the business will dictate the amount of documentation and level of detail needed.
- Identify the Structure of Documentation: this structure is applicable to all organisations regardless of its size or complexity. It includes a quality policy/objective, quality manuals, quality procedures, work instructions and quality records
- Generate QMS Documents: QMS documents detail the organisation’s structure, procedures, processes, and resources. When followed, management can be confident this will result in a quality product or service being consistently delivered to the customer. Effective documentation will assist employees in understanding the processes. As a result, enabling easier implementation and maintenance of quality records. Documents need to be easy to read, have a natural logical flow, use simple and clear language and be concise. This ensures all can understand the content and information being conveyed.
- Implement Documentation System: the document control procedure details the documentation system and how it is implemented. All documents must be controlled, legible, readily identifiable, retrievable, and available at points of use. They must be reviewed regularly for ongoing suitability throughout product/service lifecycle. Documents detail the organisation’s structure, procedures, processes, and resources. For management, the document control procedure is extremely beneficial but also critical to upkeep correctly. If documents are not effectively controlled, the organisation will very quickly run into compliance problems, and potentially require costly resources to address the problems that arise as a result.
- Maintenance and Improvement: structuring and maintaining documents in line with document control procedures to ensure organisational compliance with QMS and regulations. Documentation will be regularly reviewed and data from QMS processes evaluated to identify any changes required. Updates and improvements are identified with changes in processes, non-conformances, audits, training, identified improvements and changes to standards.
Balancing simplicity and customisation is the key to a successful QMS. There is no ‘one size fits all’ when it comes to quality management. The more detail and customisation that goes into the planning and documenting stage, the more effective and straight forward the maintenance will be, with more benefits to be gained from the entire process. Organisations need enough standardisation to produce consistent results and enough flexibility for continuous improvement to create a quality-driven culture.
Simplifying QMS Documentation
Much of what turns people away from the idea of implementing and maintaining a QMS is the notion that there needs to be complicated and extensive documentation. This is not true, in fact some of the most effective and value adding quality management systems are those with the simplest and most streamlined documentation. There is a lot to be said for documenting only what is really necessary and useful, thereby creating manuals and procedures that are truly reflective of what is happening within the organisation and are more likely to be used by all staff. The benefits of keeping documentation simple and straightforward include:
- Ensuring quality standards are routinely met
- Minimising the potential for process error
- Reducing downtime when changes occur due to being able to quickly and easily access data that is relevant
- Allowing for easy process monitoring such that the outputs are analysed and appropriate adjustments can be made
QMS documentation fulfills many functions such as communication of information, providing evidence of conformity and sharing knowledge making various types and levels of documents necessary. For small business, this requirement can sound daunting however fortunately, small businesses by their very nature will not have the volume or the complexity of processes and procedures that larger organisations often have. As each organisation is unique, they will only require documentation for the applicable quality management system requirements which apply to the organisation’s business practices.
Creating well-designed simplified documentation benefits a small business in ensuring that documentation can be easily understood and followed by employees whenever required. This results in reducing costs with:
- Ongoing procedural document maintenance
- Efficient and effective employee training
- Reduced outages and downtime due to correct and relevant documentation
- Regulatory compliance
- Audit readiness
At Southpac Certifications, we believe in doing things differently.
We know businesses are tired of the same old tick and flick approach, which is why we have built our reputation around Certification Differently – taking a fresh approach to certification.
We want the organisations we work with – both big and small – to see the benefits and success that ISO Certification can facilitate. We truly believe that effective management systems are a key enabler of that success and working with the right certification body can drive material improvements in system performance, resilience and reliability.





